Photoredox-Catalyst-Enabled para-Selective Trifluoromethylation of tert-Butyl Arylcarbamates
Yaqiqi Jiang1,+, Bao Li2,+, Nana Ma2, Sai Shu1, Yujie Chen1, Shan Yang1, Zhibin Huang1, Daqing Shi1, *(史达清), and Yingsheng Zhao1,*(赵应声)
1 Key Laboratory of Organic Synthesis of Jiangsu Province,College of Chemistry, Chemical Engineering and Materials Science,Soochow University, Suzhou 215123 (P. R. China) and School of Chemistry and Chemical Engineering Henan Normal University, Xinxiang 453000 (P. R. China)
2 Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University,Xinxiang 453000 (P. R. China)
+ These authors contributed equally to this work.
Angew. Chem. Int. Ed. 2021, 60, 19030--19034
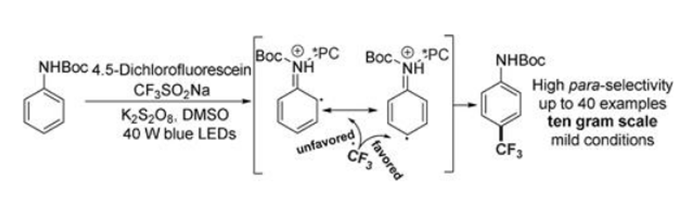
The direct incorporation of a trifluoromethyl group on an aromatic ring using a radical pathway has been extensively investigated. However, the direct highly paraselective C_H trifluoromethylation of a class of arenes has not been achieved. In this study, we report a light-promoted 4,5-dichlorofluorescein (DCFS)-enabled para-selective C_H trifluoromethylation of arylcarbamates using Langlois reagent. The preliminary mechanistic study revealed that the activated organic photocatalyst coordinated with the arylcarbamate led to para-selective C_H trifluoromethylation. Ten-gram scale reaction performs well highlighting the synthetic importance of this new protocol.
链接://onlinelibrary.wiley.com/doi/full/10.1002/anie.202105631