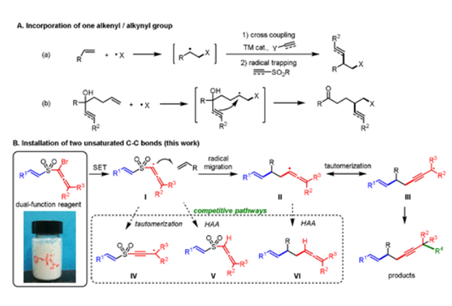
Alkene Difunctionalization Triggered by a Stabilized Allenyl Radical: Concomitant Installation of Two Unsaturated C_C Bonds
Yunlong Wei1, Hong Zhang1, Xinxin Wu1, and Chen Zhu*,1, 2(朱晨)
1 Key Laboratory of Organic Synthesis of Jiangsu Province, College of
Chemistry, Chemical Engineering and Materials Science, Soochow University,199 Ren-Ai Road, Suzhou, Jiangsu 215123 (China)
2 Key Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences,345 Lingling Road, Shanghai 200032 (China)
Angew. Chem. Int. Ed. 2021, 60, 20215--20219
Radical-mediated difunctionalization of alkenes provides a promising approach to introduce one alkenyl or alkynyl group to target compounds. However, simultaneous installation of two unsaturated C−C bonds via alkene difunctionalization remains elusive, attributable to the high instability and transient lifetimes of alkenyl and alkynyl radicals. Herein, we report the photocatalytic 1,2-alkynylalkenylation and 1,2-enynylalkenylation of alkenes for the first time, triggered by the intermolecular addition of a stabilized allenyl radical to an alkene. A portfolio of strategically designed, easily accessible dual-function reagents are applied to a radical docking-migration cascade. The protocol has broad substrate scope and efficiently increases the degree of unsaturation.
链接: //onlinelibrary.wiley.com/doi/10.1002/anie.202106145