Activation and Deactivation of Chirality Transfer in the Superbundles of Sequence-defined Stereoisomers
Zhihong Yu1, Rui Tan1(谭睿)*, Xiaoxiao Cheng1(程笑笑)*, Wei Zhang1, Yong Wang1, Jiandong Zhang1, Nianchen Zhou1(周年琛)*, Zhengbiao Zhang1,2(张正彪)*, Xiulin Zhu1
1State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Func-tional Polymer Design and Application, College of Chemistry,Chemical Engineering and Materials Science, Soochow University Suzhou, 215123, China
2State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou 215123, China
Angew. Chem. Int. Ed. 2025, 64, e202416853
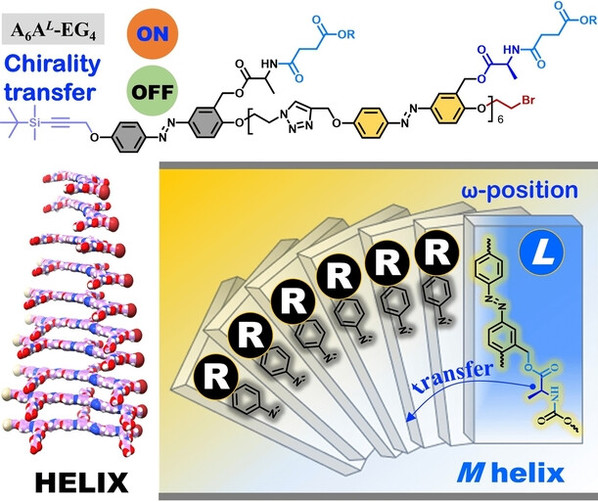
Abstract: Discrete oligomers can be used to precisely evaluate the structure–property relationship and enable unique chiroptical activities, however, the role of stereochemical sequences on chirality transfer is still unclear. Herein, we report the successful synthesis of a series of sequence-defined chiral azobenzene (Azo) oligomers via iterative stepwise chain growth strategy. Sequence-defined stereoisomers with one single chiral (L or D) stereocenter at the α-position, ω-position and middle- (m−) position have completely different self-assembly dynamics. ω-positional stereocenter can effectively command all Azo building blocks to adopt a tilted π–π stacking along the helical superbundles, exhibiting the activation of chirality transfer. However, discrete oligomers with the stereocenter at other positions can only self-assemble into non-helical nanowires, accompanied by the deactivation of chirality transfer.Cooperative supramolecular interactions, including the π–π interaction between Azo units, the intermolecular hydrogen bonding and steric hindrance, are intrinsic driving forces for these differentiations.
Article information: //doi.org/10.1002/anie.202416853