ThCTi@Cs(6)-C82: Th═C Double Bond in a Mixed Actinide-Transition Metal Cluster
ZhengkaiCao1,2, XiaojuaYu3, Yang-RongYao1,2, Jochen Autschbach1,2*,Ning Chen3(谌宁)*
1College of Chemistry, Chemical Engineering and Materials Science, and State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou,Jiangsu 215123, P. R. China
2Department of Chemistry, University at Buffalo, State University of New York, Buffalo, New York14260-3000, United States
3College of Chemistry, Chemical Engineering and Materials Science, and State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou, Jiangsu 215123, P. R. China
J. Am. Chem. Soc. 2025, 147, 3584−3592
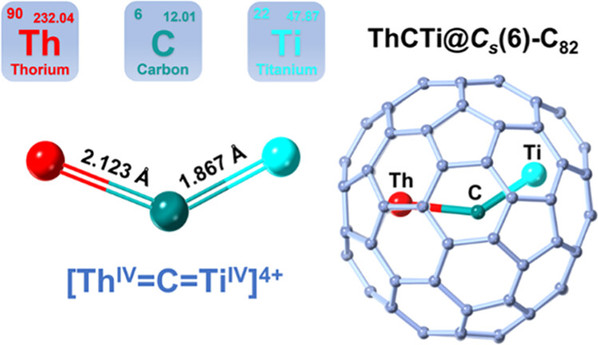
Abstract: A thorium–carbon double bond that corresponds to the sum of theoretical covalent double bond radii has long been sought after in the study of actinide-ligand multiple bonding as a synthetic target. However, the stabilization of this chemical bond remains a great challenge to date, in part because of a relatively poor energetic matching between 5f-/6d- orbitals of thorium and the 2s-/2p- frontier orbitals of carbon. Herein, we report the successful synthesis of a thorium–carbon double bond in a carbon-bridged actinide-transition metal cluster, i.e., [Th═C═Ti], encapsulated inside a fullerene cage of C82. ThCTi@Cs(6)-C82 was successfully synthesized by a modified arc discharging method and characterized by mass spectrometry, single-crystal X-ray crystallography, various spectroscopy, and theoretical calculations. X-ray crystallographic analysis reveals a bent μ2-bridged carbide cluster with a Th–C distance of 2.123(18) Å, which is the shortest reported to date in an isolable compound and is comparable to the sum of the covalent Th═C double bond radii (2.10 Å). In addition, Th═C═Ti takes an unexpected nonlinear configuration with a bond angle of 133.0(10)°. The combined experimental and theoretical investigation further revealed the bonding nature of Th═C, which is polarized toward the bridged carbon but has a notably higher covalency than the Th–C bonds reported previously for organometallic compounds. Moreover, pronounced cage-to-metal donation appears to be stabilizing the encapsulated Th═C═Ti cluster. This work offers a deeper understanding of the bonding behavior of thorium and features the unique ability of fullerene cages to stabilize bonding motifs containing different types of metal–ligand multiple bonds.
Article information: //doi.org/10.1021/jacs.4c15253